Research
Research Activities and Focus Areas
MOBL studies and collaborates on multiple areas of research interest. An important aspect of the labs’ research program is training young scientists. There’s close, day-to-day interactions between the students and the P.I. to provide guidance with research projects, manuscript and grant writing, data analysis, and presentations.
Ongoing Projects
Investigating Rotator Cuff Muscle Function Through Experimental Biomechanics and Imaging
Developing novel approaches for musculoskeletal injuries risk assessments
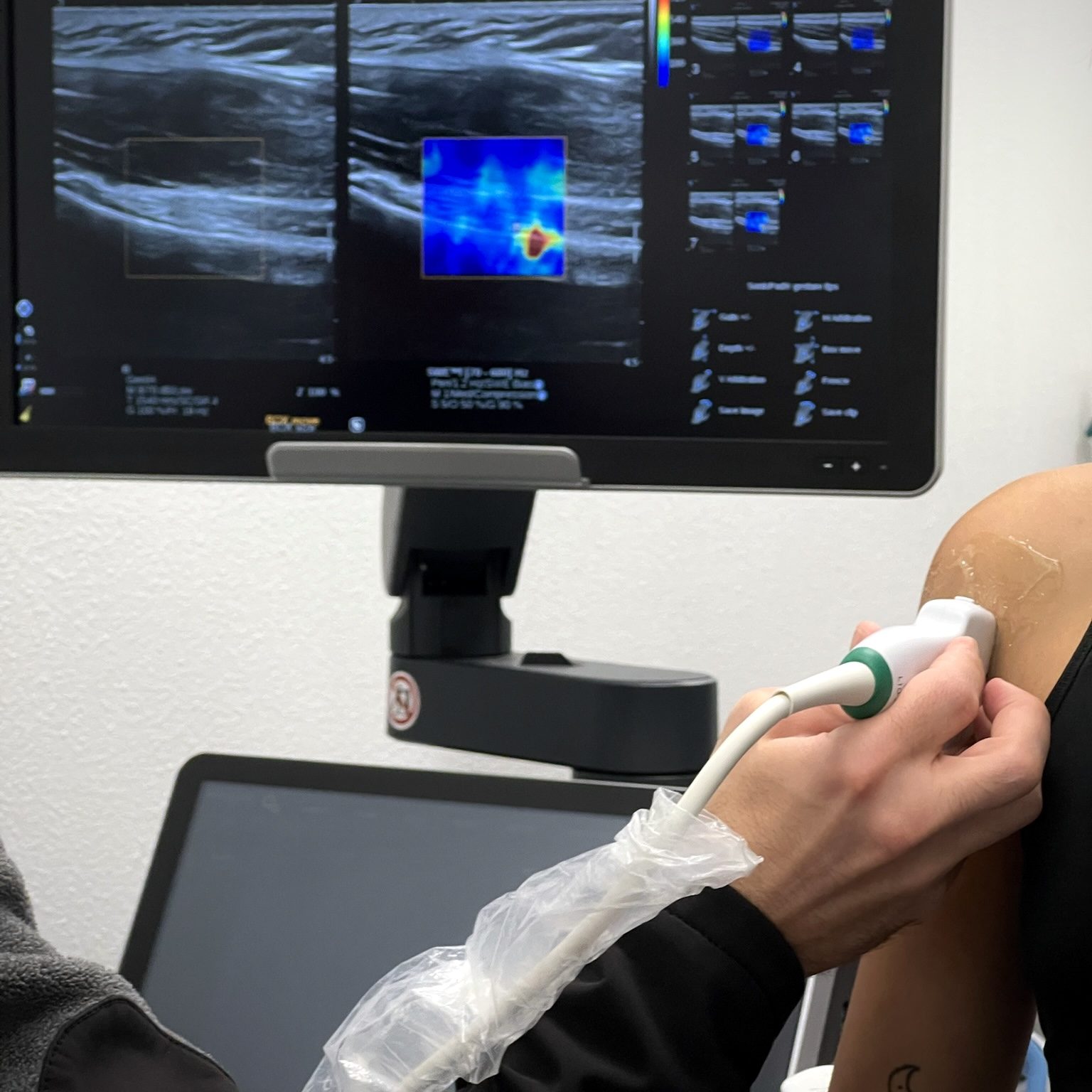
Our research focuses on improving the understanding of shoulder function and rotator cuff pathology by combining cadaveric biomechanical testing, in-vivo ultrasound shear wave elastography (SWE), and imaging-based muscle analysis. We aim to characterize the mechanical behavior and functional roles of distinct sub-regions within the rotator cuff muscles – a concept that highlights the heterogeneous nature of these muscles and their contribution to shoulder stability and movement. Through cadaveric biomechanical experiments, we assess the mechanical load-sharing properties of individual muscle sub-regions under different loading and repair conditions. Complementing this work, SWE imaging in-vivo and in cadavers allows us to quantify muscle stiffness and mechanical integrity, offering real-time insights into muscle quality and contractile function both pre- and post-operatively. By integrating these experimental techniques, our research aims to establish muscle sub-region-specific functional profiles and identify key mechanical indicators associated with rotator cuff repair failure (re-tears). Ultimately, this work will advance our ability to predict surgical outcomes, personalize rehabilitation strategies, and optimize treatment approaches based on the functional health of rotator cuff muscle sub-regions.
Our research is centered on improving vertebral fracture risk prediction by integrating quantitative computed tomography (QCT)-based finite element analysis (FEA) with musculoskeletal (MSK) whole-body modeling. This innovative approach aims to move beyond traditional bone mineral density (BMD) assessments by incorporating subject-specific bone strength estimates and loading conditions experienced during activities of daily living. Using QCT imaging, we develop detailed, patient-specific vertebral models for FEA, enabling us to evaluate accurate vertebral mechanical properties. Loads derived from MSK modeling, which simulates whole-body movement and calculates internal forces acting on the spine during activities of daily living (ADLs), such as lifting, bending, and walking, are used together with the QCT/FEA models to better predict fracture risk based on ADLs. By coupling these computational techniques, we aim to provide a more comprehensive and physiologically relevant assessment of fracture risk. This integrated framework will enhance our ability to identify individuals at high risk for fragility fractures, even when BMD alone appears normal. Ultimately, our goal is to develop personalized risk assessment tools that better reflect real-life loading conditions, improving clinical decision-making and preventative strategies for patients at risk of vertebral fractures.
Enhancing Muscle Regeneration Through Metabolites and Nanoparticle-Driven Therapeutics in Degenerative and Traumatic Conditions
Our research focuses on developing innovative regenerative medicine strategies to improve muscle repair and functional recovery following degenerative diseases and traumatic injuries, such as rotator cuff tears and ischemia reperfusion injuries. We aim to harness the therapeutic potential of novel bioactive metabolites, which play a critical role in cellular metabolism, inflammation resolution, and tissue regeneration. To optimize the delivery and bioavailability of these therapeutic metabolites, our lab, in collaboration with other groups, is implementing engineering advanced nanoparticle-based delivery systems. These nanoparticles facilitate targeting and sustained release of bioactive compounds directly to injured muscle tissues, enhancing local therapeutic effects while minimizing systemic side effects. Our interdisciplinary approach and collaborative strategies combines biomechanics, biomaterials science, cellular and molecular biology, and in-vivo models of muscle injury to evaluate the efficacy of these systems in promoting muscle regeneration. This research aims to address the limitations of current regenerative therapies by developing solutions that restore muscle structure and function in patients suffering from muscle degeneration, rotator cuff tears, and other musculoskeletal injuries.
